Watch
On-Demand
The Industry Cloud for Life Sciences
Our industry cloud solutions provide data, software, services and an extensive ecosystem of partners to support your most critical functions from R&D through commercial. Veeva helps companies of all sizes bring products to market faster and more efficiently, and maintain compliance.
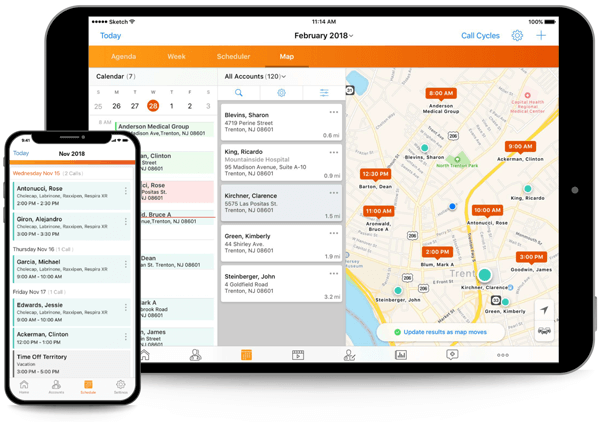
Bring Agility, Visibility, and Speed to Clinical Trials
Accelerate study timelines with modern, innovative applications.
Vault EDC
Collect, clean, and review study data
Vault Coder
Rapid coding for clinical terms
Veeva CDB
Manage complete and concurrent study data
Only suite of unified clinical operations applications on one cloud platform.
Vault Study Startup
Accelerate time to site activation
Vault eTMF
Stay inspection ready with active TMF
Vault CTMS
Enable proactive trial management
Vault Payments
Pay clinical research sites faster
Vault Study Training
Streamline and automate training
Veeva Site Connect
Automate information sharing
Veeva eConsent
Improve the patient experience
Unified RIM
Streamline your regulatory processes, improve data quality, and respond faster to business changes with Vault RIM Suite.
Vault Registrations
Manage product registration data and changes globally
Vault Submissions
Plan, track, author, review, and approve submission documents
Vault Submissions Publishing
Enable continuous publishing and speed time to submission
Vault Submissions Archive
Securely view your complete submissions history in the cloud

Learn how to get your organization's data-ready to be compliant with IDMP requirements.
Read more
Modernizing Quality Management
Seamlessly manage your quality processes, content, and training, and provide a single authoritative source of quality information.
Vault QualityDocs
Gain control of GxP documents
Vault Validation Management
Manage & execute paperless validation
Vault Station Manager
Deliver right content to the right station
Vault Training
Ensure role-based qualification & compliance
Veeva LearnGxP
Access accredited eLearning courses
Vault QMS
Manage all quality process in one place
Vault Product Surveillance
Simplify postmarket surveillance for medical devices
Vault LIMS
Optimizing QC labs for real-time batch release

Building a Business Case for Quality Management Transformation.
Read more
Are you a CDMO or Generics organization? Learn More
Real-time Management of Adverse Events
Easily intake, manage, and gain real-time oversight of adverse events and pharmacovigilance content.
Vault Safety
Collect and manage adverse event information
Vault SafetyDocs
Centrally manage all pharmacovigilance content
Vault Signal
Manage Signals from Detection through Risk Evaluation and Mitigation
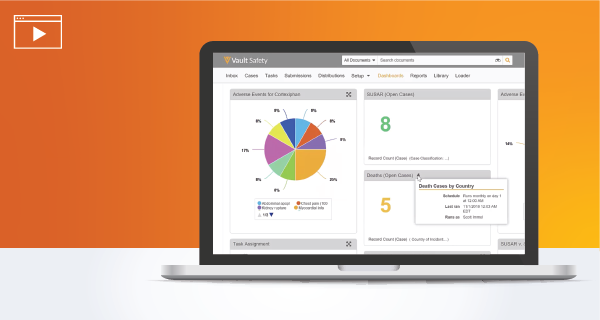
Gain visibility into safety data with real-time reports and dashboards and seamlessly collaborate with internal /external parties.
Watch the Demo
Advancing Medical Affairs
Strengthen scientific relationships, engage experts, and deliver content in one solution built for medical affairs.
Medical CRM
Foster informed scientific engagement across channels
Veeva Vault Medical
Streamline scientific communication through efficient content management and inquiry response
Veeva Link Solutions for Medical
Deepen engagement with real-time customer intelligence
A framework for delivering the right information to medical stakeholders in their preferred channel.
Read the eBook
Delivering Commercial Excellence
Connected software, data, and business consulting solutions advance
commercial excellence for sales, medical, and marketing teams.
Engagement
Veeva CRM Suite & Digital Events
Content
Vault PromoMats &
Vault MedComms
Analytics
Veeva Crossix (for U.S.) & Veeva Nitro
Customer Reference Data
Veeva OpenData
Real-time Customer Intelligence
Veeva Link
Patient & Prescriber Data
Veeva Compass (for U.S.)
Veeva Innovations Elevate Your Commercial Strategy
Watch video

"Our strategic partnership with Veeva expands our capacity to leverage innovative technology and enhances our ability to deliver value to patients and all our stakeholders – this is key to how we measure success."
, Chief Executive Officer and President, MSD
Read the Press ReleaseConnecting a Powerful Community
Become part of an active community of more than 1,100 Veeva customers
Exceed the Likely Outcome
Companies around the world are using Veeva to help change the way they do business
-
83% of new drugs approved
were launched with
Veeva CRM -
90% faster data change
request resolution with
Veeva OpenData -
40% cut in TMF
reconciliation time with
Vault eTMF -
50% reduction in submission
development time with
Vault RIM













